
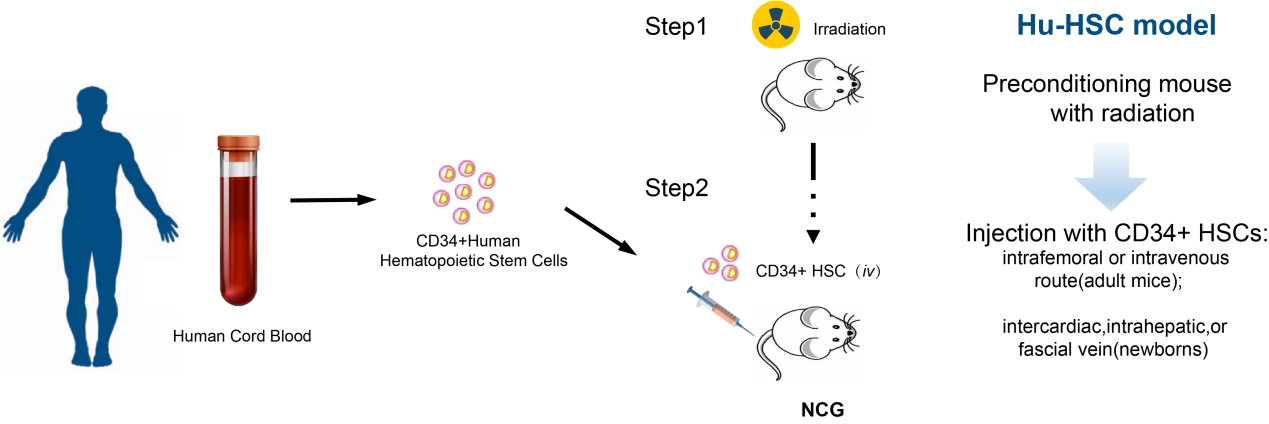
huHSC-NCG
公司基于实验动物创制策略与基因工程遗传修饰技术,为客户提供具有自主知识产权的商品化小鼠模型,同时开展模型定制、定制繁育、功能药效分析等一站式服务,满足客户在基因功能认知、疾病机理解析、药物靶点发现、药效筛选验证等基础研究和新药开发领域的实验动物小鼠模型相关需求。
huHSC-NCG小鼠是将人造血干细胞 (CD34+HSC)移植到辐照清髓的重度免疫缺陷小鼠NCG体内,并分化产生各类造血或者免疫细胞,如T细胞、B细胞以及NK细胞等,从而获得免疫系统人源化的模型以NCG小鼠为受体鼠,构建huHSC-NCG人源化小鼠。
应用领域
1. 多种肿瘤免疫治疗药物的评价
2. 免疫相关的药物评价
模型数据
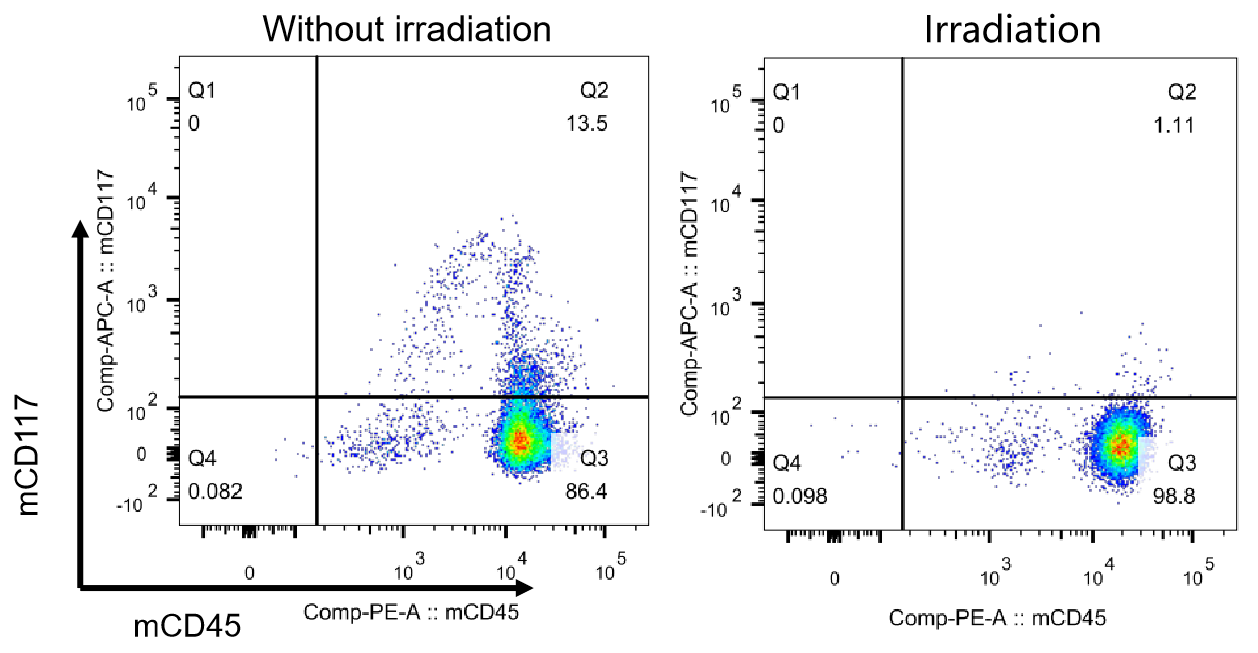
图1. NCG小鼠清髓效果检测
由于辐照可以清除小鼠骨髓中的造血干细胞,从而提高人源HSC移植后的重建水平,因此选用辐照处理后的NCG小鼠移植人源HSC。利用流式细胞技术检测辐照清髓效果。其中mCD45为小鼠白细胞标记物,mCD117为造血干细胞标记物。上图显示辐照后mCD45+mCD117+细胞群体比例为1.11%,显著低于未辐照组(13.5%)。表明150 cGys剂量辐照6-7周NCG小鼠可达到理想的清髓效果。
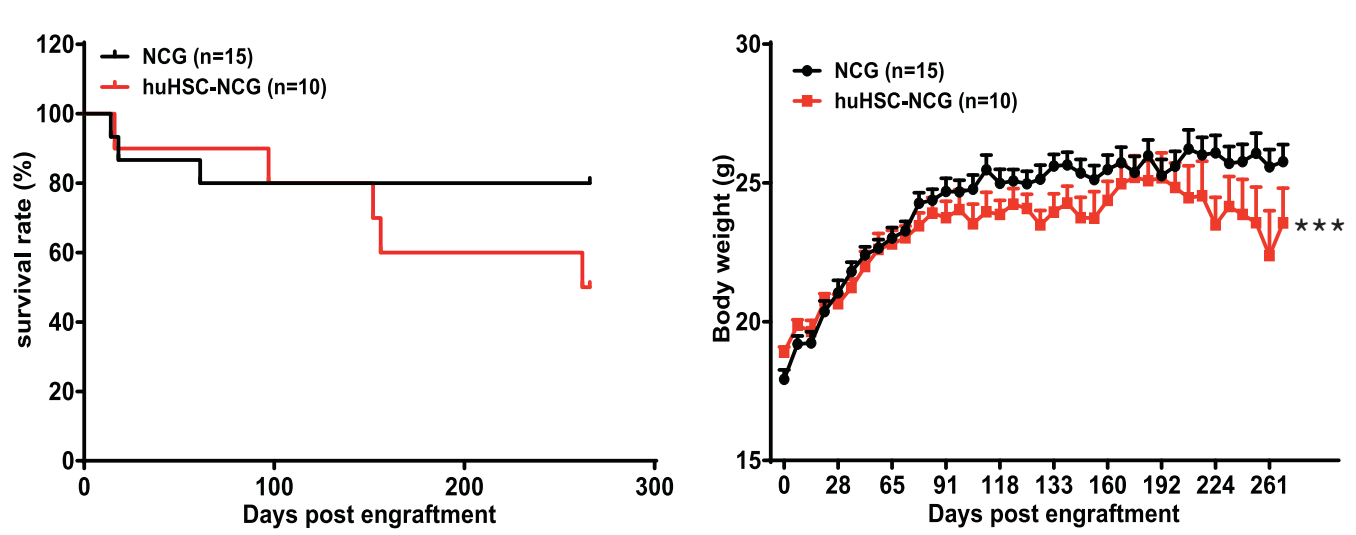
图2. huHSC-NCG小鼠的生存曲线及体重变化情况
huHSC-NCG小鼠存活时间可超过37周,且生存率与NCG野生型小鼠无显著差异。huHSC-NCG小鼠体重随时间变化呈增长趋势,前期与NCG野生型小鼠体重无显著差异,随着时间延长,huHSC-NCG小鼠体重下降,在实验终点,huHSC-NCG小鼠体重显著低于NCG野生小鼠(***, P < 0.001)。数据以Mean ± SEM形式呈现。
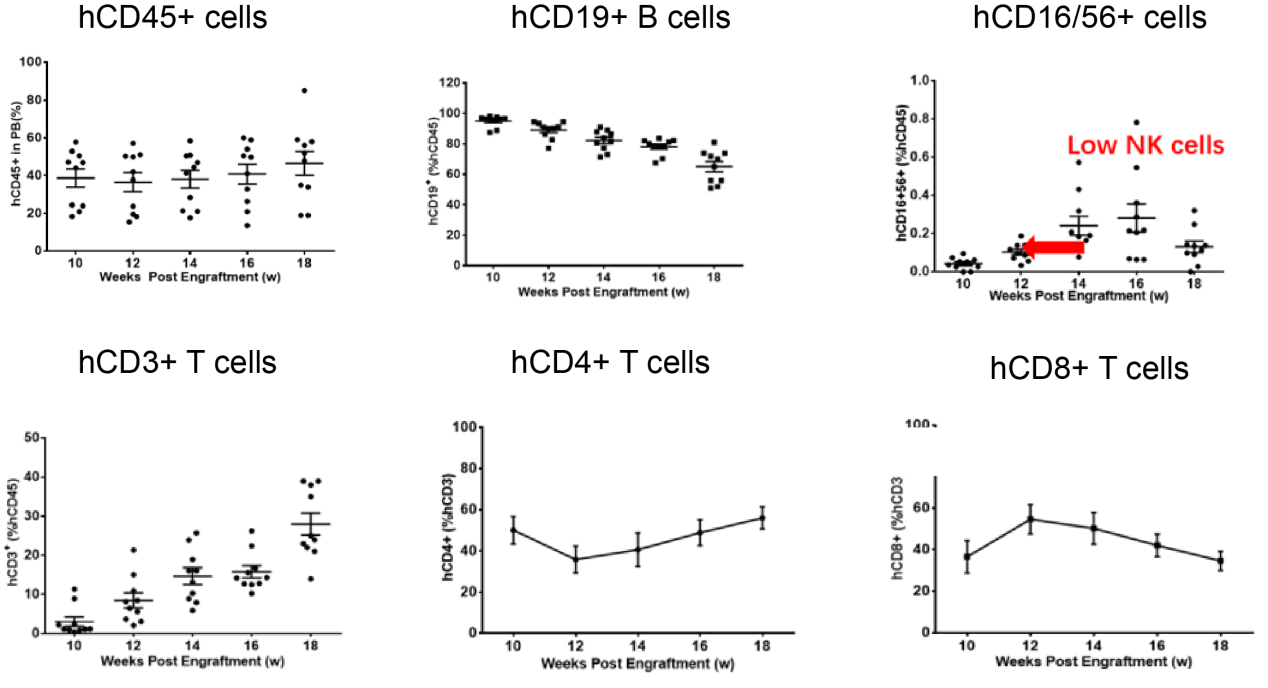
图3. huHSC-NCG重建效果鉴定
huHSC-NCG小鼠的免疫重建效率高,主要重建T细胞和大量未成熟的B细胞,以及少量NK细胞。
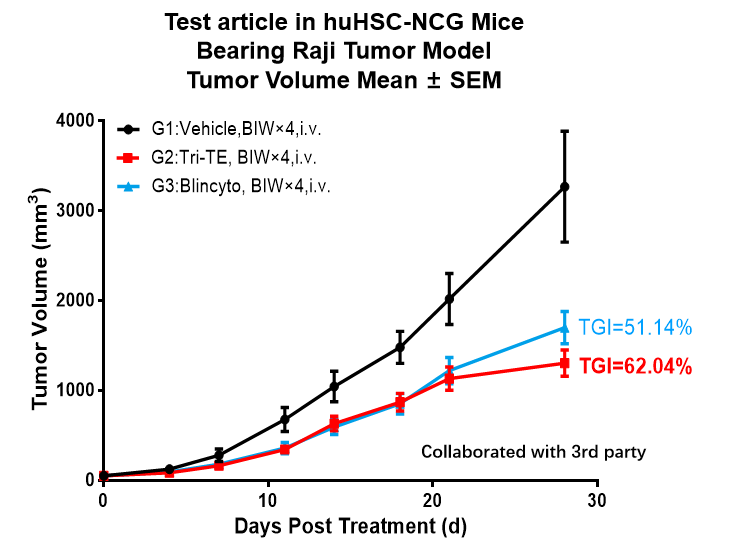
图4. 基于huHSC-NCG小鼠模型的体内药效评价
基于huHSC-NCG小鼠体内用药试验。将对数生长期人淋巴瘤细胞Raji细胞接种到huHSC-NCG小鼠皮下,待肿瘤生长至平均体积约40-50 mm3时,根据小鼠肿瘤体积和体重,随机分为Vehicle组,Tri-TE给药组,Blincyto给药组,并使用相应的药物进行治疗。结果显示:Tri-TE组(TGI=62.04%)及Blincyto给药组(TGI=51.14%)对huHSC-NCG的Raji细胞荷瘤鼠上肿瘤生长有抑制作用。
NCG在CAR-T治疗上的应用
CAR-T(Chimeric Antigen Receptor T Cell)是肿瘤免疫治疗的新方法。CAR-T细胞具有靶向识别肿瘤抗原的能力,其杀伤肿瘤细胞无需进行抗原递呈,与以往免疫疗法相比,具有特异性高、攻击持久等优势。在恶性肿瘤,特别是血液肿瘤治疗中有很好的疗效。集萃药康具有丰富的CDX和自主知识产权的PDX库,包括多种实体瘤和血液瘤,为CAR-T药效评价提供了丰富的肿瘤模型资源。同时,集萃药康开发了外周血中CAR-T细胞计数方法和细胞因子检测方法,满足CAR-T实验检测需求。
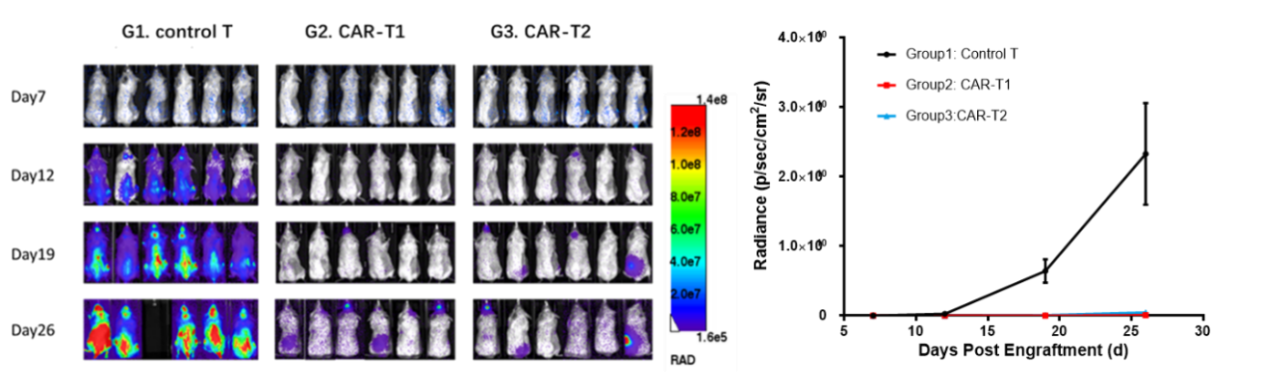
图5.在NCG小鼠上接种Nalm6-Luciferase后,进行CAR-T药效评价
Nalm6-Luciferase细胞尾静脉接种到NCG小鼠后,第7天根据肿瘤负荷进行分组,分别给予Control T,CAR-T1及CAR-T2细胞治疗,并于D12,D19,D26利用活体成像技术检测肿瘤负荷。据体内成像及统计结果显示,与Control T相比,CAR-T1和CAR-T2细胞均能很好的抑制小鼠体内肿瘤生长,延长小鼠生存期。
参考文献
1.
Hu B, Yu M, Ma X, et al. Interferon-a potentiates anti-PD-1 efficacy by
remodeling glucose metabolism in the hepatocellular carcinoma
microenvironment. Cancer discovery. Apr 12
2022;doi:10.1158/2159-8290.Cd-21-1022(IF:39.397)
2. Ma W, Yang Y, Zhu J, et al. Biomimetic Nanoerythrosome‐Coated Aptamer‐DNA Tetrahedron/Maytansine Conjugates: pH‐Responsive and Targeted Cytotoxicity for HER2‐positive Breast Cancer. Advanced Materials.2109609.(IF:30.849)
3. Song H, Liu D, Wang L, et al. Methyltransferase like 7B is a potential therapeutic target for reversing EGFR-TKIs resistance in lung adenocarcinoma. Molecular cancer. Feb 10 2022;21(1):43. (IF:41.444)
4. Zhang L, Zhu Z, Yan H, et al. Creatine promotes cancer metastasis through activation of Smad2/3. Cell metabolism. 2021;33(6):1111-1123. e4.(IF:22.4)
5. Liu C, Zou W, Nie D, et al. Loss of PRMT7 reprograms glycine metabolism to selectively eradicate leukemia stem cells in CML. Cell Metab. Apr 26 2022.(IF:22.4)
6. Zhang X-N, Yang K-D, Chen C, et al. Pericytes augment glioblastoma cell resistance to temozolomide through CCL5-CCR5 paracrine signaling. Cell Research. 2021:1-16. (IF:25.617)
7. Dai Z, Mu W, Zhao Y, et al. T cells expressing CD5/CD7 bispecific chimeric antigen receptors with fully human heavy-chain-only domains mitigate tumor antigen escape. Signal transduction and targeted therapy. Mar 25 2022;7(1):85.(IF:18.187)
8. Dai Z, Liu H, Liao J, et al. N7-Methylguanosine tRNA modification enhances oncogenic mRNA translation and promotes intrahepatic cholangiocarcinoma progression. Molecular Cell. 2021. (IF:17.97)
9. Hao M, Hou S, Li W, et al. Combination of metabolic intervention and T cell therapy enhances solid tumor immunotherapy. Science Translational Medicine. 2020;12(571).(IF:17.956)
10. Liu Y, Liu G, Wang J, et al. Chimeric STAR receptors using TCR machinery mediate robust responses against solid tumors. Science Translational Medicine. 2021;13(586).(IF:17.956)
11. Yan H, Wang Z, Sun Y, Hu L, Bu P. Cytoplasmic NEAT1 Suppresses AML Stem Cell Self‐Renewal and Leukemogenesis through Inactivation of Wnt Signaling. Advanced Science. 2021;8(22):2100914. (IF:17.521)
12. Luo Q, Wu X, Chang W, et al. ARID1A prevents squamous cell carcinoma initiation and chemoresistance by antagonizing pRb/E2F1/c-Myc-mediated cancer stemness. Cell Death & Differentiation. 2020;27(6):1981-1997. (IF:12.067)
13. Wu M, Zhang X, Zhang W, et al. Cancer stem cell regulated phenotypic plasticity protects metastasized cancer cells from ferroptosis. Nature communications. Mar 16 2022;13(1):1371.(IF:14.919)
14. Liu H, Bai L, Huang L, et al. Bispecific antibody targeting TROP2xCD3 suppresses tumor growth of triple negative breast cancer. Journal for ImmunoTherapy of Cancer. 2021;9(10):e003468.(IF:12.469)

